
| Technique(s) used for Glycosylation Detection | Schiff's staining, peroxidase-conjugated concanavalin A (ConA)-binding |
| Technique(s) used for Glycosylated Residue(s) Detection | N-terminal amino acid sequencing coupled with fast atom bombardment-mass spectrometry (FAB-MS), Edman degradation on a gas-phase sequencer, FAB-MS |
| Protein Glycosylation- Implication | 1. The presence of the mannose residues on the Apa protein was essential for the antigenicity of the molecules in T-cell-dependent immune responses in vitro (proliferation assay) and in vivo (delayed type hypersensitivity or DTH reaction). The deglycosylated antigen was 10-fold less active than native molecules in eliciting DTH. Mannosylation of antigen leads to selective targeting and subsequent greater presentation by dendritic cells.
2. In Mtb, mannosylated nApa is a potential adhesin and has a role in host cell attachment, entry, and immune evasion. |
| Glycan Information |
| Glycan Annotation | Linkage: αMan-Thr.
34.6% sugar detected.
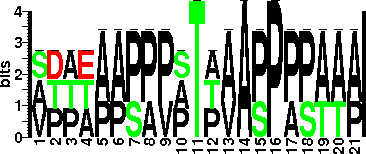
α-D-Manp(1→2)α-D-Manp (mannobiose), α-D-Manp (single mannose), α-D-Manp(1→2)α-D-Manp(1→2)α-D-Manp (mannotriose) are present. T10 and T18 are glycosylated with mannobiose, T27 with single mannose, and T277 with either of the three. The majority of the antigen species bear six, seven, or eight mannose residues (22, 24, and 17%, respectively), while others three, four, or five mannoses (5, 9, and 14%, respectively). Alpha-D-Man, mannobiose, or mannotriose, Thr residues at positions 10 and 18 were substituted a-D-Manp(132)a-D-Manp, position 27 was substituted with a single a-D-Manp, Thr-277 was substituted with either a-D-Manp, a-D-Manp(132)a-D-Manp, or a-D-Manp(132)a-D-Manp
(132)a-D-Manp. |
| Technique(s) used for Glycan Identification | GC-MS of partially methylated alditol acetates obtained by trifluoro acetic acid (TFA) hydrolysis of permethylated oligoglycosyl alditols which are released by elimination of the glycopeptides. |
| Protein Glycosylation linked (PGL) gene(s) |
| OST Gene Name | Protein O-mannosyltransferase |
| OST ProGT ID | ProGT12 |
| Characterized Accessory Gene(s) | Polyprenol-phosphate-mannose (PPM) synthase, Ppm1, is present. A second type of Ppm synthase (Rv3779 gene product) exclusive to slow-growing mycobacteria, is a membrane glycosyltransferase. It mannosylates polyprenyl-phosphates directly from GDP-mannose. |
| Accessory Gene(s)Progt ID | ProGT10.1 |
| Additional Comment | Sec-mediated translocation influences the O-mannosylation. Ppm1 does not discriminate between polyprenol substrates with variable chain lengths and saturation of the isoprene units.
Glycosylation sites have been found to be located within proline-rich domains (or Thr-rich sequences) near the N-terminus and the C-terminus.
In a cell-free assay, the M. smegmatis mannosyltransferase activity in membrane and cell wall fraction has been shown to catalyze transfer of radiolabeled mannose from GDP-[14C]mannose to peptide acceptors. The acceptors consisted of Thr-rich sequences from M. tuberculosis 45 kDa antigen (Ref. no. 3).
The 45/47 kDa antigen (Apa) has also been glycosylated in Streptomyces lividans. The 45- and 47-kDa represent two glycoforms of the antigen (Ref. no. 2). |
| Literature |
| Year of Identification | 1989 |
| Year of Identification Month Wise | 1989.09.01 |
| Year of Validation | 1996 |
| Reference | Scherman, H., Kaur, D., Pham, H., Škovierová, H., Jackson, M. and Brennan, P.J., 2009. Identification of a polyprenylphosphomannosyl synthase involved in the synthesis of mycobacterial mannosides. Journal of bacteriology, 191(21), pp.6769-6772. |
| Corresponding Author | Patrick J Brennan |
| Contact | Department of Microbiology, Colorado State University, Fort Collins, Colorado 80523-1682, USA. |
| Reference | Lara, M., Servín-González, L., Singh, M., Moreno, C., Cohen, I., Nimtz, M. and Espitia, C., 2004. Expression, secretion, and glycosylation of the 45-and 47-kDa glycoprotein of Mycobacterium tuberculosis in Streptomyces lividans. Applied and Environmental Microbiology, 70(2), pp.679-685. |
| Corresponding Author | Clara Espitia |
| Contact | Department of Immunology, Institute of Biomedical Research, National Autonomous University of Mexico, Mexico D.F., Mexico. |
| Reference | Cooper, H.N., Gurcha, S.S., Nigou, J., Brennan, P.J., Belisle, J.T., Besra, G.S. and Young, D., 2002. Characterization of mycobacterial protein glycosyltransferase activity using synthetic peptide acceptors in a cell-free assay. Glycobiology, 12(7), pp.427-434. |
| Corresponding Author | Douglas Young |
| Contact | Centre for Molecular Microbiology and Infection, Imperial College of Science, Technology and Medicine, South Kensington, London, SW7 2AZ, England. |
| Reference | Horn, C., Namane, A., Pescher, P., Riviere, M., Romain, F., Puzo, G., Bârzu, O. and Marchal, G., 1999. Decreased capacity of recombinant 45/47-kDa molecules (Apa) of Mycobacterium tuberculosis to stimulate T lymphocyte responses related to changes in their mannosylation pattern. Journal of Biological Chemistry, 274(45), pp.32023-32030. |
| Corresponding Author | Gilles Marchal |
| Contact | Department of Pathophysiology of Infection, Pasteur Institute, 25 rue du Dr. Roux, 75724 Paris Cedex 15, France. |
| Reference | Romain, F., Horn, C., Pescher, P., Namane, A., Riviere, M., Puzo, G., Barzu, O. and Marchal, G., 1999. Deglycosylation of the 45/47-kilodalton antigen complex of Mycobacterium tuberculosis decreases its capacity to elicit in vivo or in vitro cellular immune responses. Infection and immunity, 67(11), pp.5567-5572. |
| Corresponding Author | Gilles Marchal |
| Contact | Department of Pathophysiology of Infection, Pasteur Institute, 25 rue du Dr. Roux, 75724 Paris Cedex 15, France. |
| Reference | Dobos, K.M., Khoo, K.H., Swiderek, K.M., Brennan, P.J. and Belisle, J.T., 1996. Definition of the full extent of glycosylation of the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. Journal of bacteriology, 178(9), pp.2498-2506. |
| Corresponding Author | JOHN T. BELISLE |
| Contact | Department of Microbiology, Colorado State University, Fort Collins 80523, USA. |
| Reference | Mehaffy, C., Belisle, J.T. and Dobos, K.M., 2019. Mycobacteria and their sweet proteins: An overview of protein glycosylation and lipoglycosylation in M. tuberculosis. Tuberculosis, 115, pp.1-13. |
| Corresponding Author | Carolina Mehaffy |
| Contact | Department of Microbiology, Immunology and Pathology, Colorado State University, 1682 Campus delivery, Fort Collins, CO, USA |
| Reference | Nandakumar, S., Kannanganat, S., Dobos, K.M., Lucas, M., Spencer, J.S., Fang, S., McDonald, M.A., Pohl, J., Birkness, K., Chamcha, V. and Ramirez, M.V., 2013. O-mannosylation of the Mycobacterium tuberculosis adhesin Apa is crucial for T cell antigenicity during infection but is expendable for protection. PLoS pathogens, 9(10), p.e1003705. |
| Corresponding Author | Suraj B. Sable |
| Contact | Division of Tuberculosis Elimination, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia, United States of America. |
| Reference | Espitia, C. and Mancilla, R. (1989) Identification, isolation and partial characterization of Mycobacterium tuberculosis glycoprotein antigens. Clin Exp Immunol, 77, 378-383. [PubMed: 2478323] |
| Contact | Department of Immunology, National Autonomous University of Mexico, D.F |