
| Technique(s) used for Glycosylation Detection | ESMS (electrospray mass spectrometry); 10%- 6.5 kDa- mass excess detected |
| Technique(s) used for Glycosylated Residue(s) Detection | A combination of LC-ESMS (liquid chromatography-electrospray mass spectrometry) and MS-MS (tandem mass spectrometry) analyses; 10 out of 19 glycosylation sites were defined using nano-ESMS after base (NH4OH)-catalyzed β-elimination. |
| Protein Glycosylation- Implication | Glycosylation is required for flagellar filament formation. Certain glycans mediate filament-filament interactions resulting in AAG (autoagglutination) and other glycans appear to be critical for structural subunit-subunit interactions within the filament. Modification with pseudaminic acid and derivatives is essential for targeting and/or secretion of flagellin. Also, specific structural modifications to the flagellin glycoform have been shown to be involved in the biological fitness of C. jejuni in colonization of chickens. |
| Glycan Information |
| Glycan Annotation | Glycan represents 10% of the total mass of the protein.
Major glycan is pseudaminic acid and its derivatives, Pse5Pr7Pr, Pse5Ac7Ac8OAc, Pse5Am7Ac.
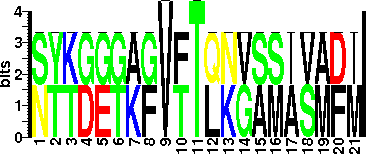
Pse5Ac7Ac (5,7-diacetamido-3,5,7,9 - tetradeoxy-L-glycero-L-manno- nonulosonic acid), with 5-acetamidino pseudaminic acid (Pse5Am7Ac) and 5,7-N-(2,3-dihydroxyproprionyl)-pseudaminic acid (Pse5Pr7Pr) are also present. In addition, novel glycans, Pse5Am7Ac8GlnAc and Pse5Ac7Ac8OAc, have also been found. S398 and S405 carry Pse5Pr7Pr moiety while T394, S401 and S409 are modified with Pse5Ac7Ac residues. Pse5Ac7Ac is also written as Pse5NAc7NAc (Pse). Microheterogeneity in glycosylation has been observed. |
| Technique(s) used for Glycan Identification | Nano-ESMS and NMR analysis of HPLC fractions of trypsin digested glycopeptides including COSY(correlated spectroscopy) and NOESY (nuclear Overhauser effect spectroscopy). |
| Protein Glycosylation linked (PGL) gene(s) |
| OST ProGT ID | ProGT18 (PseD) |
| Literature |
| Reference | Maita, N., Nyirenda, J., Igura, M., Kamishikiryo, J. and Kohda, D., 2010. Comparative Structural Biology of Eubacterial and Archaeal Oligosaccharyltransferases 2. Journal of Biological Chemistry, 285(7), pp.4941-4950. |
| Corresponding Author | Daisuke Kohda |
| Contact | Division of Structural Biology, Medical Institute of Bioregulation, Kyushu University, Maidashi 3-1-1, Higashi-ku, Fukuoka 812-8582, Japan. |
| Reference | Ewing, C.P., Andreishcheva, E. and Guerry, P., 2009. Functional characterization of flagellin glycosylation in Campylobacter jejuni 81-176. Journal of bacteriology, 191(22), pp.7086-7093. |
| Corresponding Author | Patricia Guerry |
| Contact | NRC-Institute for Biological Sciences, Ottawa, Canada. |
| Reference | McNally, D.J., Hui, J.P., Aubry, A.J., Mui, K.K., Guerry, P., Brisson, J.R., Logan, S.M. and Soo, E.C., 2006. Functional characterization of the flagellar glycosylation locus in Campylobacter jejuni 81–176 using a focused metabolomics approach. Journal of Biological Chemistry, 281(27), pp.18489-18498. |
| Corresponding Author | Evelyn C. Soo |
| Contact | National Research Council, Institute for Biological Sciences, Ottawa, Ontario K1A 0R6, Canada. |
| Reference | Goon, S., Kelly, J.F., Logan, S.M., Ewing, C.P. and Guerry, P., 2003. Pseudaminic acid, the major modification on Campylobacter flagellin, is synthesized via the Cj1293 gene. Molecular microbiology, 50(2), pp.659-671. |
| Corresponding Author | Patricia Guerry |
| Contact | NRC-Institute for Biological Sciences, Ottawa, Canada. |
| Reference | Logan, S.M., Kelly, J.F., Thibault, P., Ewing, C.P. and Guerry, P., 2002. Structural heterogeneity of carbohydrate modifications affects serospecificity of Campylobacter flagellins. Molecular microbiology, 46(2), pp.587-597. |
| Corresponding Author | Patricia Guerry |
| Contact | NRC-Institute for Biological Sciences, Ottawa, Canada. |
| Reference | Thibault, P., Logan, S.M., Kelly, J.F., Brisson, J.R., Ewing, C.P. and Guerry, P., 2001. Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. Journal of Biological Chemistry, 276(37), pp.34862-34870. |
| Corresponding Author | Susan M Logan |
| Contact | Institute for Biological Sciences, National Research Council of Canada, Ottawa, Ontario K1A 0R6, Canada. |